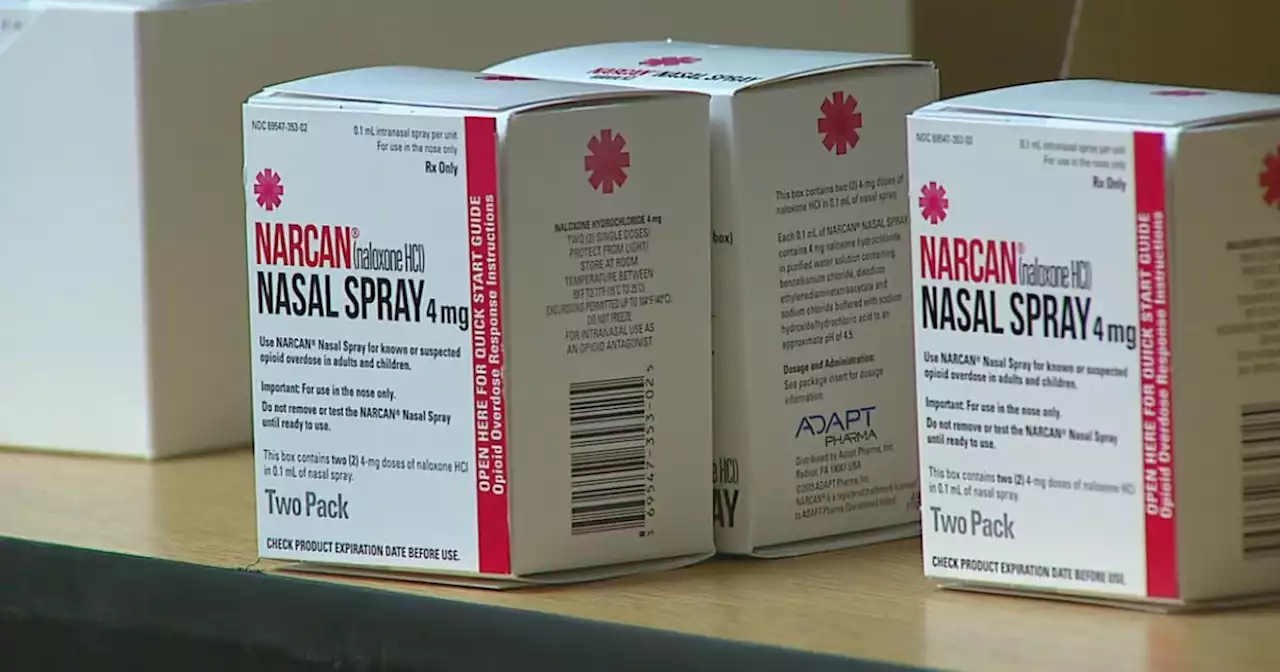
FDA to give priority review to nonprescription form of naloxone, a life-saving drug used to treat suspected drug overdoses.
Americans may soon no longer need a prescription to buy a life-saving nasal spray for people who overdose on opioids.
Emergent BioSolutions on Tuesday said the Food and Drug Administration has fast-tracked its application for an over-the-counter version of Narcan, a nasal-spray form of naloxone. Approved in 2015, Narcan and its somewhat less expensive generic competitors are widely used by first responders and laypeople to treat known or suspected opioid overdoses that kill tens of thousands of Americans in the U.S. each year.
The FDA's priority review means the agency could approve the spray to be sold in drugstores in late March of next year, the Gaithersburg, Maryland-based company stated in a news "We are taking to this step to help address the rising and devastating number of opioid overdoses and fatalities happening across the country," Emergent CEO Robert Kramer said in a statement.
More than 105,000 Americans died of drug overdoses during the 12-month period ending in October 2021, with 66% of those deaths related to synthetic opioids like fentanyl, according
Malaysia Latest News, Malaysia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA to review Emergent's request to approve over-the-counter NarcanShares of Emergent BioSolutions Inc. undefined were up 1.7% in premarket trading on Tuesday after the company said the Food and Drug Administration is...
FDA to review Emergent's request to approve over-the-counter NarcanShares of Emergent BioSolutions Inc. undefined were up 1.7% in premarket trading on Tuesday after the company said the Food and Drug Administration is...
Read more »
 Over-the-Counter Narcan to Reverse Overdoses May Be Available Early Next YearEmergent BioSolutions said Tuesday that the FDA will decide whether to approve its OTC nasal spray by late March 2023.
Over-the-Counter Narcan to Reverse Overdoses May Be Available Early Next YearEmergent BioSolutions said Tuesday that the FDA will decide whether to approve its OTC nasal spray by late March 2023.
Read more »
 WSJ News Exclusive | Narcan Maker Gets Fast-Tracked for Over-the-Counter Nasal SprayThe market for over-the-counter opioid-reversal drugs is heating up, with Narcan maker’s application for approval getting priority review from the FDA
WSJ News Exclusive | Narcan Maker Gets Fast-Tracked for Over-the-Counter Nasal SprayThe market for over-the-counter opioid-reversal drugs is heating up, with Narcan maker’s application for approval getting priority review from the FDA
Read more »
 Ex-FDA vaccine inspectors call for better trainingTraining in the Food and Drug Administration’s office that oversees licensed vaccines has decreased dramatically in recent years, raising concerns that the team is not equipped to identify quality control issues in manufacturing
Ex-FDA vaccine inspectors call for better trainingTraining in the Food and Drug Administration’s office that oversees licensed vaccines has decreased dramatically in recent years, raising concerns that the team is not equipped to identify quality control issues in manufacturing
Read more »