The U.S. Food and Drug Administration has updated its mammography regulations, which affect physicians and medical centers across the country.
The new regulations will also"strengthen the FDA's oversight and enforcement of facilities and help interpreting physicians better categorize and assess mammograms," the agency"Today's action represents the agency's broader commitment to support innovation to prevent, detect and treat cancer," said Dr. Hilary Marston, the FDA's chief medical officer."Since 1992, the FDA has worked to ensure patients have access to quality mammography.
. However, according to the institute, there is no link between dense breasts and death from breast cancer. According to the FDA, 1 in 8 women will have breast cancer in their life. The FDA changes are amendments to regulations issued under a 1992 law that gives the FDA oversight over mammography facilities, but it's not until now that the FDA has used the law to require facilities to inform women if they have dense breast tissue.
"While nearly all certified mammography facilities continue to meet quality standards, today's updates, among other things, enhance the FDA's ability to communicate directly, if needed, with patients and their health care providers in cases where a facility did not meet quality standards and is not adequately communicating with patients about its deficiencies," the FDA said in a news release.
Malaysia Latest News, Malaysia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Albuterol Shortage Challenges Hospitals Treating Patients With Breathing ProblemsHospitals are running out of a liquid form of albuterol, a medication used to treat people with breathing problems, weeks after the only maker of the product declared bankruptcy
Albuterol Shortage Challenges Hospitals Treating Patients With Breathing ProblemsHospitals are running out of a liquid form of albuterol, a medication used to treat people with breathing problems, weeks after the only maker of the product declared bankruptcy
Read more »
 FDA: Two more eyedrop brands recalled due to risksU.S. health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and serious injury.
FDA: Two more eyedrop brands recalled due to risksU.S. health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and serious injury.
Read more »
 FDA: Two more eyedrop brands recalled due to risksU.S. health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and serious injury
FDA: Two more eyedrop brands recalled due to risksU.S. health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and serious injury
Read more »
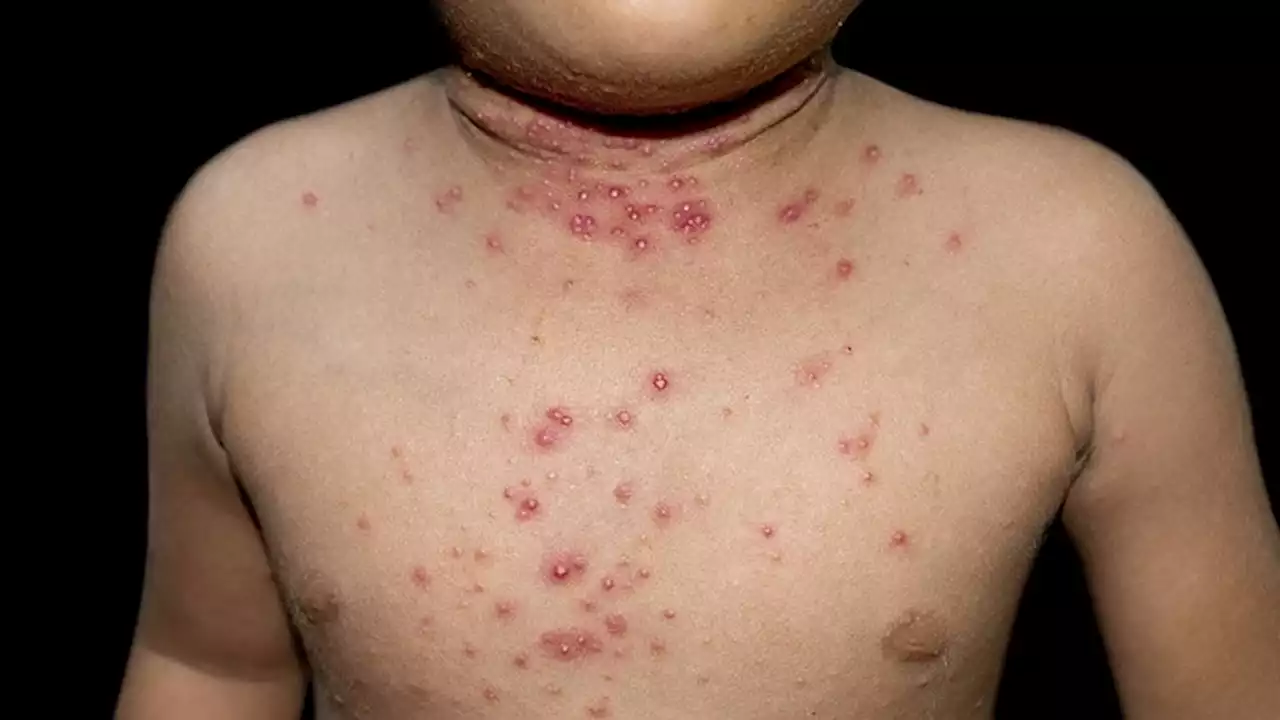 FDA Accepts Application for Topical Molluscum TreatmentIf approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States.
FDA Accepts Application for Topical Molluscum TreatmentIf approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States.
Read more »
 FDA: Two more eyedrop brands recalled due to risksU.S. health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and serious injury.
FDA: Two more eyedrop brands recalled due to risksU.S. health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and serious injury.
Read more »
 FDA Warns Consumers About Two More Eyedrop Brand RecallsThis is the second time health officials have warned consumers about recalls of eyedrops due to contamination risks this year.
FDA Warns Consumers About Two More Eyedrop Brand RecallsThis is the second time health officials have warned consumers about recalls of eyedrops due to contamination risks this year.
Read more »
