In a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
U.S. women getting mammograms will soon receive information about their breast density because of new government rules.regulations will require mammography facilities to notify patients about the density of their breasts.
"Today's action represents the agency's broader commitment to support innovation to prevent, detect and treat cancer," said Dr. Hilary Marston, the FDA's chief medical officer. But it shows up as white on a mammogram, making cancer -- which also appears white -- more difficult to detect, according to the American Cancer Society. Dense breasts are also associated with up to a four times higher risk of breast cancer.
The new changes require facilities to provide patients with information about their breast density and include specific language in the mammogram reports to explain how breast density can influence the accuracy of a mammogram. The changes, which amend the Mammography Quality Standards Act of 1992, are required to be implemented within 18 months. The FDA says these new regulations will enhance inspection of mammography practices and communication with patients and providers.
Malaysia Latest News, Malaysia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
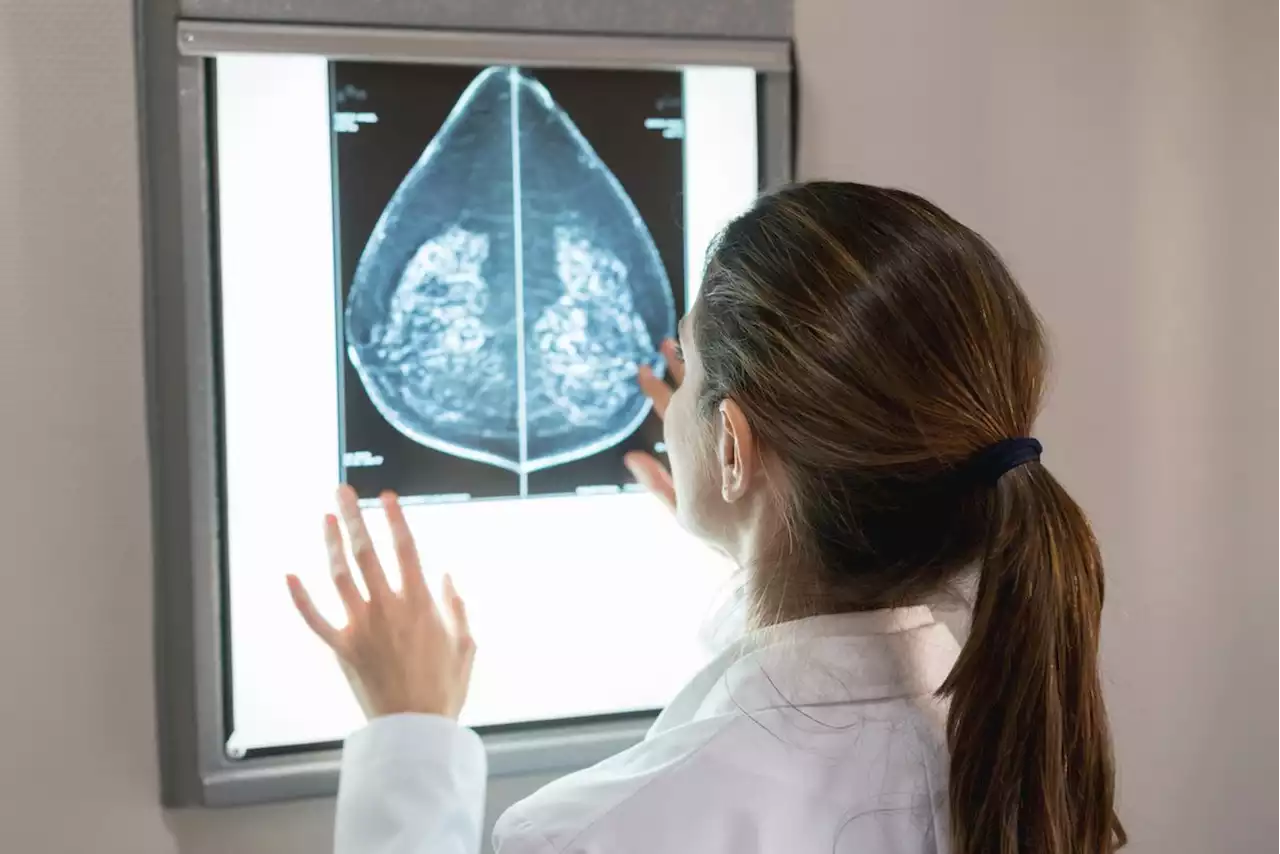 FDA Orders New Mammogram Standards for Women With Dense BreastsWomen with dense breast tissue will be given additional information at their cancer screenings under new rules adopted by the FDA. Providers of mammograms will have to tell women that their tests are harder to interpret and suggest they speak with their doctors about more testing.
FDA Orders New Mammogram Standards for Women With Dense BreastsWomen with dense breast tissue will be given additional information at their cancer screenings under new rules adopted by the FDA. Providers of mammograms will have to tell women that their tests are harder to interpret and suggest they speak with their doctors about more testing.
Read more »
 Mammogram facilities must notify women if they have dense breast tissue, FDA new guidances sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Mammogram facilities must notify women if they have dense breast tissue, FDA new guidances sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Read more »
 Mammogram facilities must notify women if they have dense breast tissue, FDA new guidances sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Mammogram facilities must notify women if they have dense breast tissue, FDA new guidances sayIn a major effort to improve breast cancer prevention, the FDA will now require mammogram facilities to inform women if they have dense breast tissue.
Read more »
 FDA to require mammogram reports include breast density information | CNNThe US Food and Drug Administration announced updates to mammography regulations on Thursday, including requiring mammography facilities to notify patients about the density of their breasts.
FDA to require mammogram reports include breast density information | CNNThe US Food and Drug Administration announced updates to mammography regulations on Thursday, including requiring mammography facilities to notify patients about the density of their breasts.
Read more »
 FDA issues new mammogram regulations aimed at further breast cancer preventionThe FDA has released updated regulations that require mammogram providers to notify patients about the density of their breast tissue, which can increase breast cancer risk.
FDA issues new mammogram regulations aimed at further breast cancer preventionThe FDA has released updated regulations that require mammogram providers to notify patients about the density of their breast tissue, which can increase breast cancer risk.
Read more »
