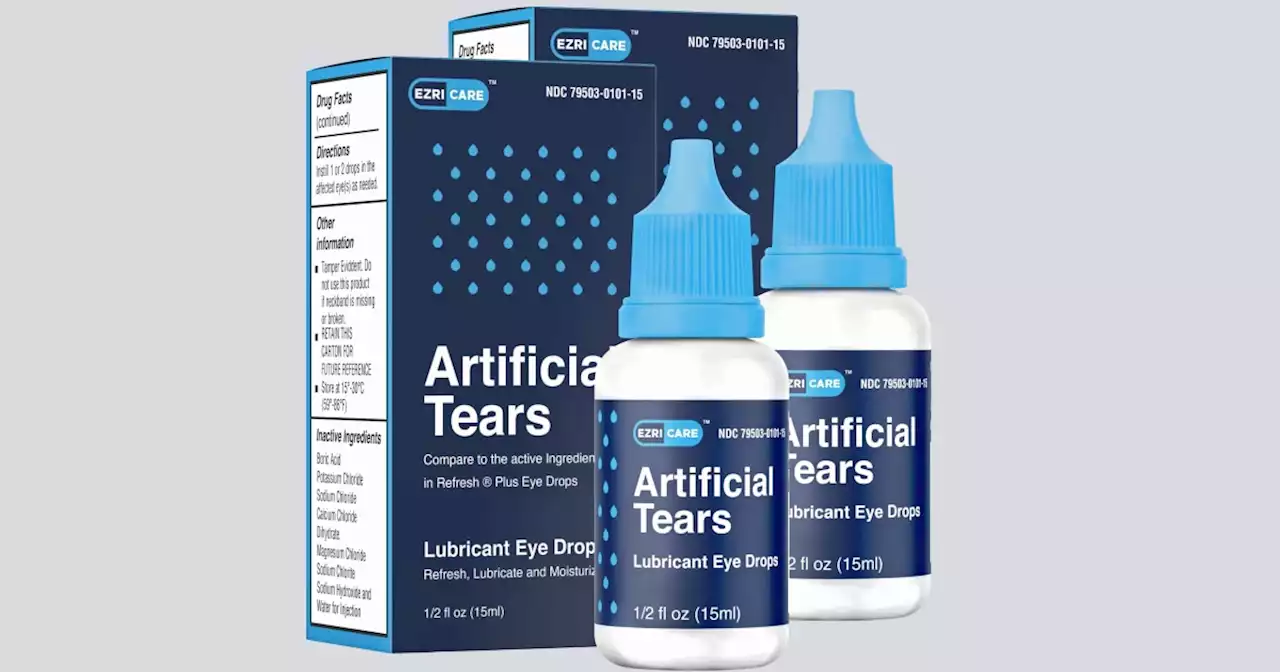
The manufacturer of eyedrops recently linked to deaths and injuries lacked measures to assure sterility at its factory in India, according to U.S. health inspectors.
Inspectors arrived at the plant Feb. 20, more than two weeks after the announcement of the first eyedrop recall on Feb. 3. The inspection appears to be the FDA’s first visit to the plant, according to agency records.
“We urge consumers to stop using these products which may be harmful to their health,” FDA’s Jeremy Khan wrote in an emailed statement. FDA inspectors cited worrisome sanitary conditions at the Global Pharma plant, noting that its floors, walls and ceilings were not “easily cleanable.” At one point during the visit, an FDA inspector noted “none of the equipment on the filling machine was wrapped or covered.” The inspector also noted the company didn’t have rigorous procedures for ensuring bottles were fully sealed. Instead, a “manual visual inspection is the only test to detect any leak,” according to the report.
Malaysia Latest News, Malaysia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops
Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops
Read more »
 Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops. FDA inspectors found about a dozen problems with how Global Pharma Healthcare made and tested the drops. The FDA released its preliminary inspection report Monday. Recalled eyedrops made at the plant have been linked to 68 bacterial infections in the U.S., including three deaths and eight cases of vision loss. Four people have had to have their eyeballs surgically removed due to infection. The outbreak is considered particularly worrisome because the bacteria driving it is resistant to standard antibiotics.
Eyedrops maker couldn't ensure factory was sterile, FDA saysThe Food and Drug Administration says it found a host of problems at an Indian manufacturing plant recently linked to contaminated eyedrops. FDA inspectors found about a dozen problems with how Global Pharma Healthcare made and tested the drops. The FDA released its preliminary inspection report Monday. Recalled eyedrops made at the plant have been linked to 68 bacterial infections in the U.S., including three deaths and eight cases of vision loss. Four people have had to have their eyeballs surgically removed due to infection. The outbreak is considered particularly worrisome because the bacteria driving it is resistant to standard antibiotics.
Read more »
 Yerba Buena Art and Maker's Market Kicks Off in San FranciscoThe Yerba Buena Art and Maker’s Market kicked off Sunday at the Yerba Buena Gardens in San Francisco. The event gave space for creators to perform and sell their goods.
Yerba Buena Art and Maker's Market Kicks Off in San FranciscoThe Yerba Buena Art and Maker’s Market kicked off Sunday at the Yerba Buena Gardens in San Francisco. The event gave space for creators to perform and sell their goods.
Read more »
 Taste Test: Maker’s Mark’s New Limited Bourbon Exaggerates the Core Whiskey’s Best FlavorsIf you're a fan of Maker's Mark, you won't want to miss their latest limited-edition bourbon. This BEP (barrel entry proof) amplifies the balanced wood sugars, making it a must-try for any whiskey connoisseur. 🥃 whiskey MakersMark
Taste Test: Maker’s Mark’s New Limited Bourbon Exaggerates the Core Whiskey’s Best FlavorsIf you're a fan of Maker's Mark, you won't want to miss their latest limited-edition bourbon. This BEP (barrel entry proof) amplifies the balanced wood sugars, making it a must-try for any whiskey connoisseur. 🥃 whiskey MakersMark
Read more »
 Micron Technology: China probes US chip maker for cybersecurity risks as tech tension escalates | CNN BusinessChina has launched a cybersecurity probe into Micron Technology, one of America's largest memory chip makers, in apparent retaliation after US allies in Asia and Europe announced new restrictions on the sale of key technology to Beijing.
Micron Technology: China probes US chip maker for cybersecurity risks as tech tension escalates | CNN BusinessChina has launched a cybersecurity probe into Micron Technology, one of America's largest memory chip makers, in apparent retaliation after US allies in Asia and Europe announced new restrictions on the sale of key technology to Beijing.
Read more »
 EV maker Rivian misses first-quarter production estimates; shares dropRivian Automotive Inc on Monday missed first-quarter production estimates as the electric-vehicle maker grappled with supply chain constraints, sending its shares down 4% in early trade.
EV maker Rivian misses first-quarter production estimates; shares dropRivian Automotive Inc on Monday missed first-quarter production estimates as the electric-vehicle maker grappled with supply chain constraints, sending its shares down 4% in early trade.
Read more »