PUTRAJAYA, April 7 — The Ministry of Health (MoH) has agreed to grant additional indication approval for the product Evusheld 100 milligrammes per millimetre (mg/ml) Solution...
PUTRAJAYA, April 7 — The Ministry of Health has agreed to grant additional indication approval for the product Evusheld 100 milligrammes per millimetre Solution for Injection manufactured by Astrazeneca AB of Sweden.
Health director-general Tan Sri Dr Noor Hisham Abdullah said the approval was made at the 383rd Drug Control Authority meeting which convened yesterday. Earlier, the 372nd meeting agreed to grant conditional registration approval for the same product. The conditional approval status remains and the latest approval is for additional indications for the treatment of Covid-19.
Evusheld is used to treat adult and adolescent Covid-19 patients weighing at least 40 kilogrammes who do not require oxygen support and are at high risk for more severe coronavirus infection, he said in a statement today. It is also used on individuals who may not have an adequate immune response to Covid-19 vaccination or for individuals for whom Covid-19 vaccination is not recommended.Advertisement
Malaysia Latest News, Malaysia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Health Ministry approves use of Evusheld medication for Covid-19 preventionPUTRAJAYA: The Health Ministry has agreed to grant additional indication approval for the product Evusheld 100mg/ml solution for injection (Tixagevimab 100mg/ml and Cilgavimab 100mg/ml) manufactured by Astrazeneca AB of Sweden.
Health Ministry approves use of Evusheld medication for Covid-19 preventionPUTRAJAYA: The Health Ministry has agreed to grant additional indication approval for the product Evusheld 100mg/ml solution for injection (Tixagevimab 100mg/ml and Cilgavimab 100mg/ml) manufactured by Astrazeneca AB of Sweden.
Read more »
 KKM lulus produk Evusheld rawatan Covid-19Evusheld digunakan untuk merawat pesakit Covid-19 yang tidak memerlukan bantuan oksigen dan berisiko tinggi mendapat jangkitan lebih teruk.
KKM lulus produk Evusheld rawatan Covid-19Evusheld digunakan untuk merawat pesakit Covid-19 yang tidak memerlukan bantuan oksigen dan berisiko tinggi mendapat jangkitan lebih teruk.
Read more »
 MOH approves additional indication of Evusheld to prevent Covid-19 infectionPUTRAJAYA: The Ministry of Health (MOH) has agreed to grant additional indication approval for the product Evusheld 100 milligrammes per millimetre (m...
MOH approves additional indication of Evusheld to prevent Covid-19 infectionPUTRAJAYA: The Ministry of Health (MOH) has agreed to grant additional indication approval for the product Evusheld 100 milligrammes per millimetre (m...
Read more »
 Health Ministry approves new NF1 drug with 'orphan medicine' statusPUTRAJAYA: The Health Ministry has agreed to give 'orphan medicine’ status to Koselugo Hard Capsules 10 milligrammes (mg) and 25 mg, said Health director-general Tan Sri Dr Noor Hisham Abdullah.
Health Ministry approves new NF1 drug with 'orphan medicine' statusPUTRAJAYA: The Health Ministry has agreed to give 'orphan medicine’ status to Koselugo Hard Capsules 10 milligrammes (mg) and 25 mg, said Health director-general Tan Sri Dr Noor Hisham Abdullah.
Read more »
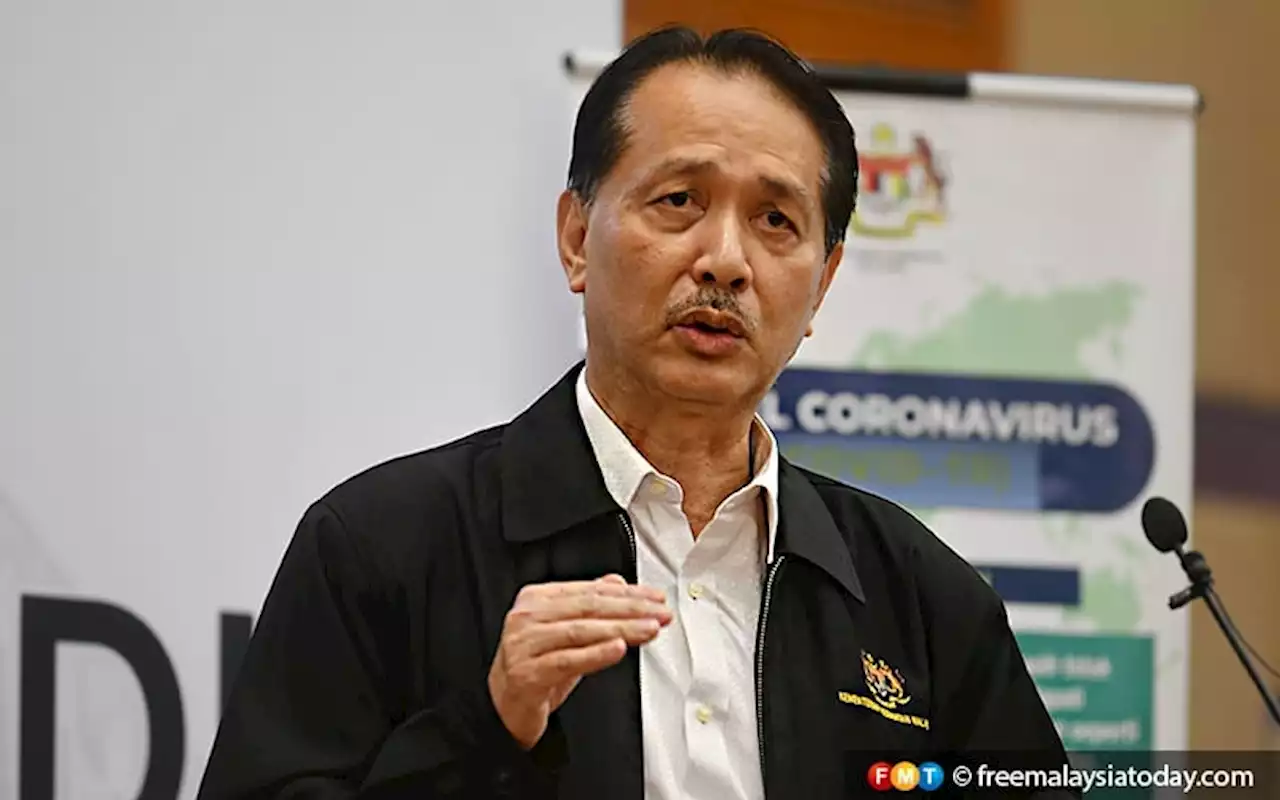 Any claims on organ-selling must be probed, says health ministryHealth director-general Dr Noor Hisham Abdullah says the buying and selling of organs is a form of exploitation of vulnerable groups and a violation of medical ethics.
Any claims on organ-selling must be probed, says health ministryHealth director-general Dr Noor Hisham Abdullah says the buying and selling of organs is a form of exploitation of vulnerable groups and a violation of medical ethics.
Read more »
 Amid organ trafficking concerns, Health Ministry says transplant committee set up to monitor donationsKUALA LUMPUR, April 7 — The Ministry of Health (MoH) has established a transplant committee to monitor organ donations among living donors involving unrelated donor-recipient...
Amid organ trafficking concerns, Health Ministry says transplant committee set up to monitor donationsKUALA LUMPUR, April 7 — The Ministry of Health (MoH) has established a transplant committee to monitor organ donations among living donors involving unrelated donor-recipient...
Read more »
