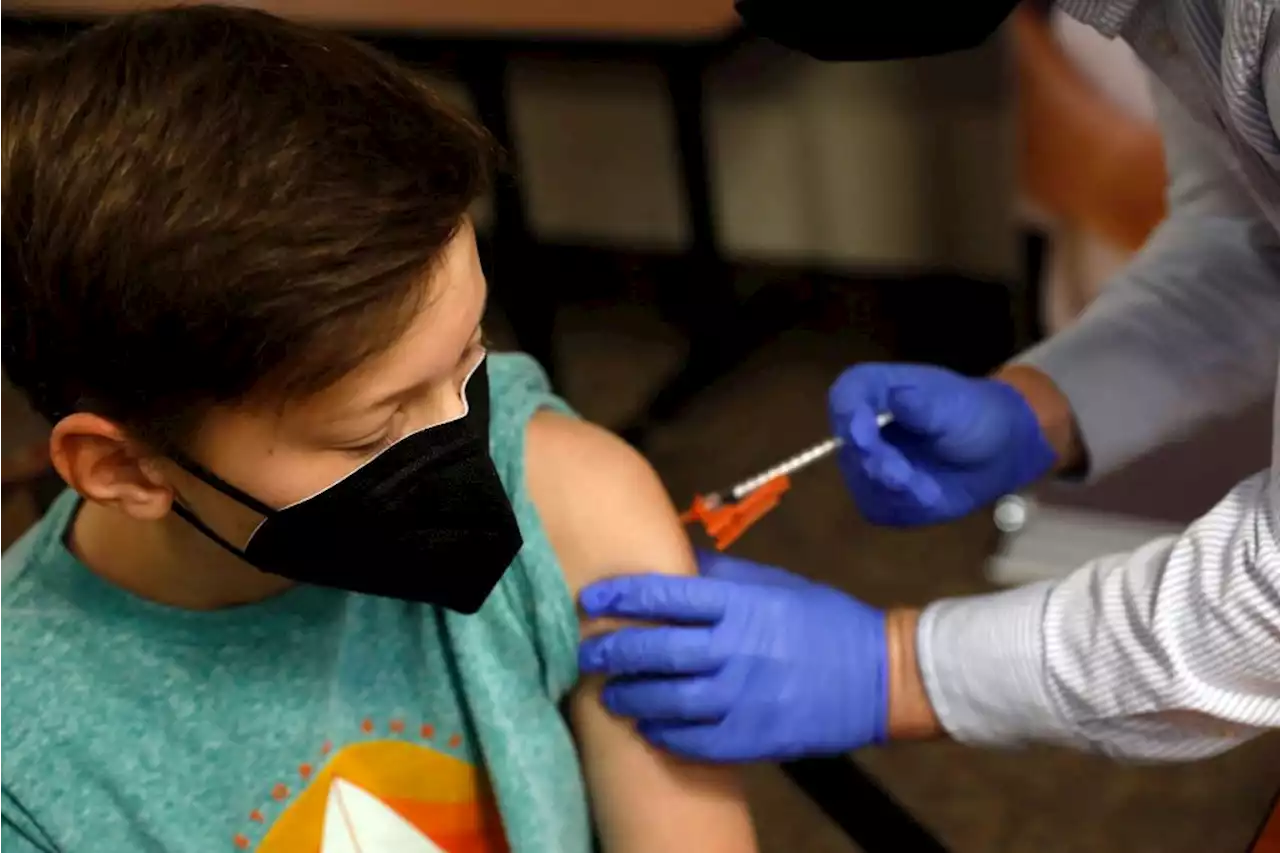
FDA Committee Delays Review of Pfizer-BioNTech's COVID-19 Vaccine for Young Kids
postponed a meeting to discuss the authorization of Pfizer-BioNTech’s COVID-19 vaccine
The FDA’s Vaccines and Related Biological Products Advisory Committee was scheduled to meet on Feb. 15 to discuss emergency use authorization of a two-dose regimen of Pfizer-BioNTech’s vaccine formulated for children ages 6 months through 4 years. But on Feb. 11, the FDA announced that Pfizer had notified the agency of new data from its ongoing clinical trial testing a three-dose regimen for kids, necessitating a longer review period.
Malaysia Latest News, Malaysia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
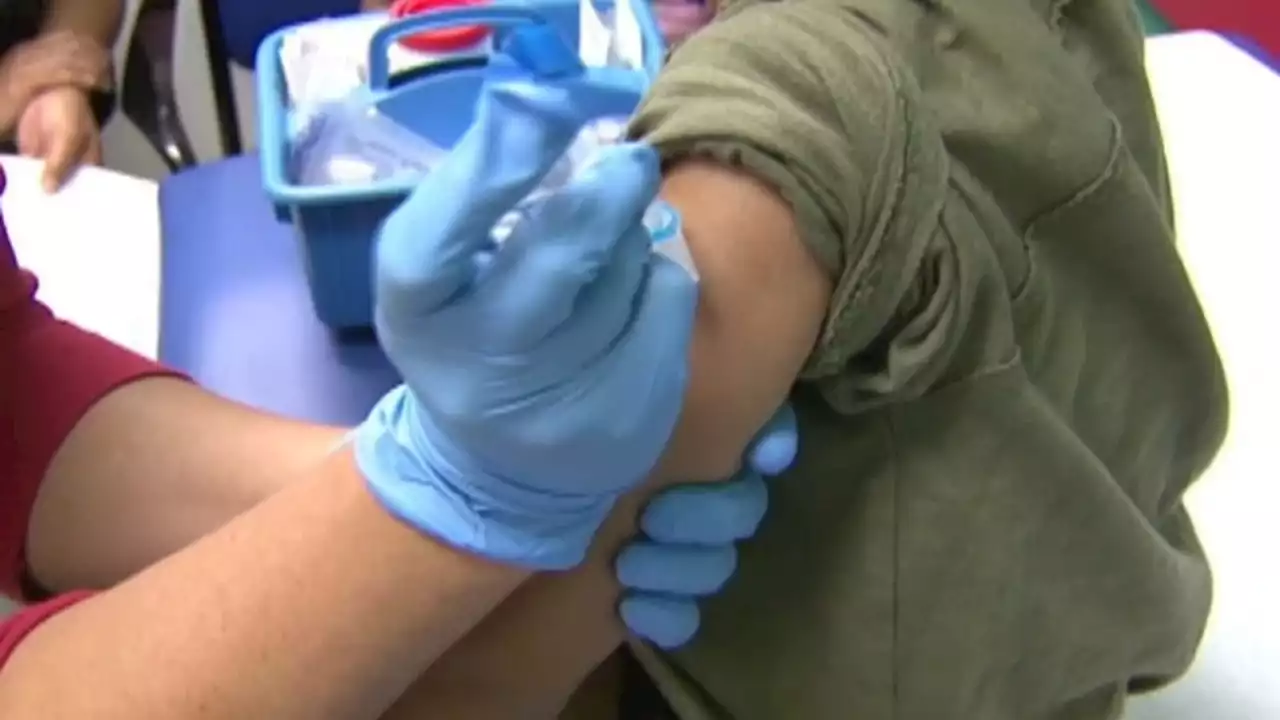 FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataBREAKING: The FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataBREAKING: The FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
Read more »
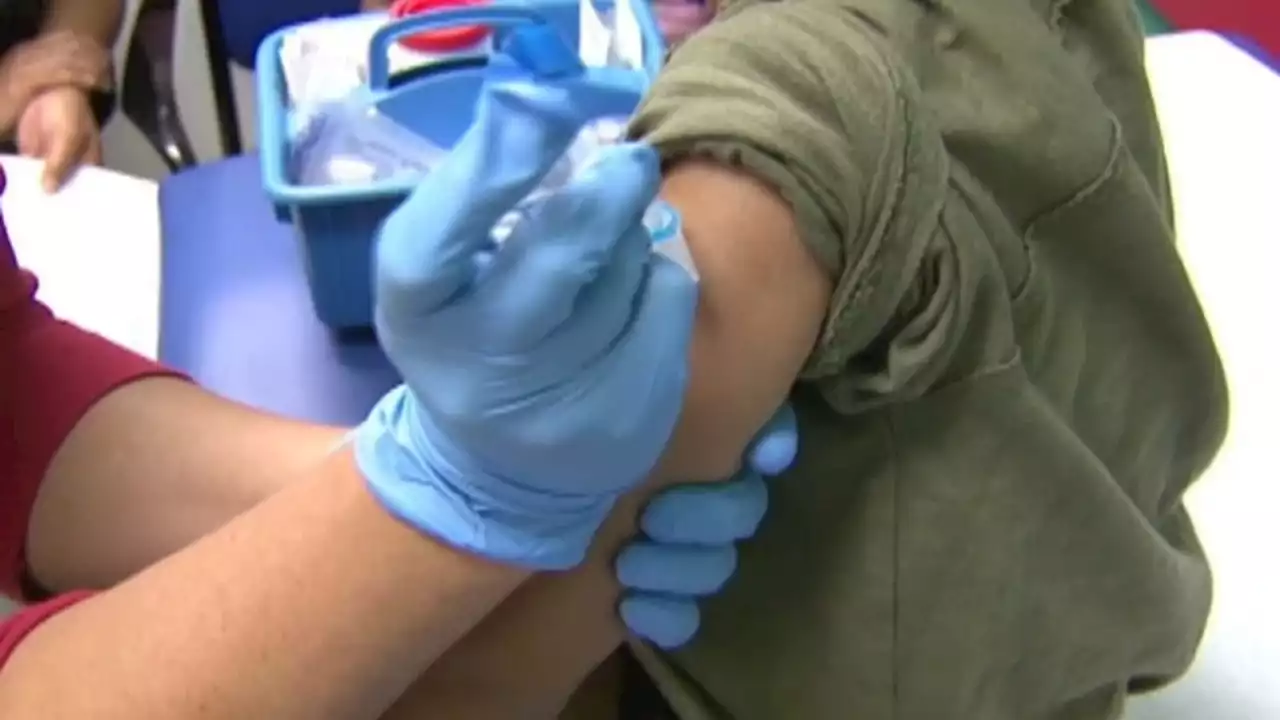 FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
Read more »
 FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
FDA delays public meeting for COVID-19 vaccine for children under 5 to review more dataThe FDA has delayed a public meeting for a COVID-19 vaccine for children under 5 in order to review more data.
Read more »
 FDA delays meeting on kid vaccines as Pfizer promises more dataThe companies have been testing a third shot after announcing in December that trial data showed two doses produced an insufficient immune response in toddlers.
FDA delays meeting on kid vaccines as Pfizer promises more dataThe companies have been testing a third shot after announcing in December that trial data showed two doses produced an insufficient immune response in toddlers.
Read more »
 FDA Committee Votes Against Eli Lilly Cancer Treatment Over Concerns Trials Conducted Only in ChinaFDA committee members said the trial population of mostly Asian men did not represent the diversity of U.S. patients.
FDA Committee Votes Against Eli Lilly Cancer Treatment Over Concerns Trials Conducted Only in ChinaFDA committee members said the trial population of mostly Asian men did not represent the diversity of U.S. patients.
Read more »