U.S. regulators on Tuesday authorized a COVID-19 booster shot for healthy 5- to 11-year-olds, hoping an extra vaccine dose will enhance their protection as infections once again creep upward.
There is one more hurdle: The Centers for Disease Control and Prevention must decide whether to formally recommend the booster for this age group. The CDC’s scientific advisers are scheduled to meet on Thursday.
Whether elementary-age children need a booster has been overshadowed by parents’ outcry to vaccinate even younger tots, those under 5 -- the only group not yet eligible in the U.S. Both Pfizer and rival Moderna have been studying their shots in the youngest children, and the FDA is expected to evaluate data from one or both companies sometime next month.
But in a small study, Pfizer found a booster revved up those kids’ levels of virus-fighting antibodies -- including those able to fight omicron -- the same kind of jump adults get from an extra shot. Adding to public confusion, the CDC estimates 3 out of every 4 U.S. children of all ages have been infected with the coronavirus since the pandemic’s start -- many of them during the winter omicron wave. Still, health authorities urge vaccination even in people who’ve previously had COVID-19, to strengthen their protection.
Malaysia Latest News, Malaysia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
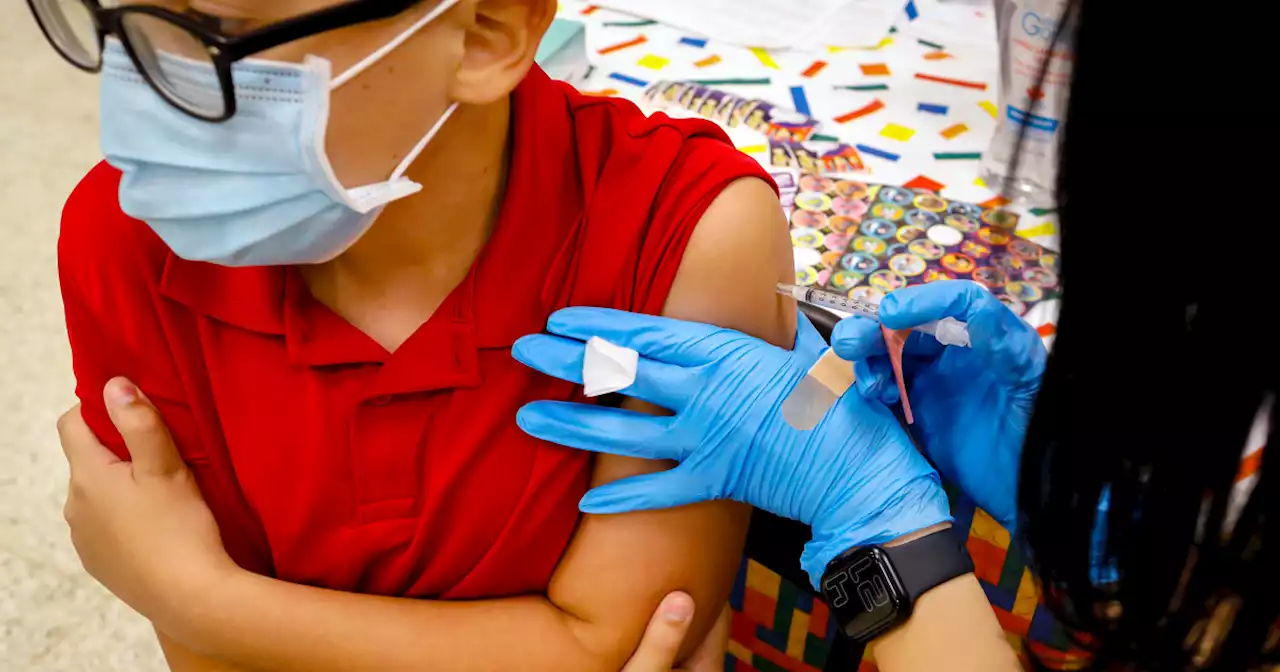 FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
Read more »
 FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
Read more »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Read more »
 FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
FDA approves Pfizer COVID-19 booster for children ages 5 to 11Pfizer’s COVID-19 booster shot for U.S. kids ages 5 to 11 has been approved by the FDA. Next, it will need the CDC’s final sign-off.
Read more »
 FDA authorizes COVID-19 booster for children ages 5 to 11The Food and Drug Administration (FDA) amended Pfizer's COVID-19 booster shot emergency use authorization to allow for children ages 5 to 11 to receive the vaccine.
FDA authorizes COVID-19 booster for children ages 5 to 11The Food and Drug Administration (FDA) amended Pfizer's COVID-19 booster shot emergency use authorization to allow for children ages 5 to 11 to receive the vaccine.
Read more »
