FDA has approved injectable poly-L-lactic acid for the correction of fine lines and wrinkles in the cheek, the manufacturer announced today. AntiAging FineLineFighter
The US Food and Drug Administration has approved injectable poly-L-lactic acid for the correction of fine lines and wrinkles in the cheek, the manufacturer announced today.
With this expanded approval, PLLA-SCA is now indicated for use in people with healthy immune systems for correcting shallow-to-deep nasolabial fold contour deficiencies, fine lines, and wrinkles in the cheeks and other facial areas., PLLA-SCA achieved the primary efficacy endpoint of at least a 1-grade improvement in wrinkles on both cheeks concurrently at rest and its secondary endpoint of improving cheek wrinkles when smiling for up to 2 years, the company states.
The most common side effects after initial treatment are injection site swelling, tenderness, redness, pain, bruising, bleeding, itching, and lumps, according to the company. Other side effects may include small lumps under the skin that are sometimes noticeable when pressing on the treated area.
Malaysia Latest News, Malaysia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA grants accelerated approval to Biogen’s treatment for rare form of ALSThe U.S. Food and Drug Administration said Tuesday it has granted accelerated approval to Biogen Inc.’s torferson, a treatment for a rare form of amyotrophic...
FDA grants accelerated approval to Biogen’s treatment for rare form of ALSThe U.S. Food and Drug Administration said Tuesday it has granted accelerated approval to Biogen Inc.’s torferson, a treatment for a rare form of amyotrophic...
Read more »
 FDA grants accelerated approval to Biogen treatment for rare form of ALSThe FDA has deemed Biogen's torferson reasonably likely to produce a clinical benefit for patients.
FDA grants accelerated approval to Biogen treatment for rare form of ALSThe FDA has deemed Biogen's torferson reasonably likely to produce a clinical benefit for patients.
Read more »
 FDA Approves Drug for Rare Form of Lou Gehrig's DiseaseApproval came via FDA’s accelerated pathway, which allows drugs to launch based on promising early results, before they’re confirmed to benefit patients.
FDA Approves Drug for Rare Form of Lou Gehrig's DiseaseApproval came via FDA’s accelerated pathway, which allows drugs to launch based on promising early results, before they’re confirmed to benefit patients.
Read more »
 FDA approves new drug for rare form of ALSThe FDA has approved a new drug to treat a rare form of amyotrophic lateral sclerosis, or ALS. The drug is expected to help people with a very specific mutation, SOD1, which applies to about 2% of the ALS population.
FDA approves new drug for rare form of ALSThe FDA has approved a new drug to treat a rare form of amyotrophic lateral sclerosis, or ALS. The drug is expected to help people with a very specific mutation, SOD1, which applies to about 2% of the ALS population.
Read more »
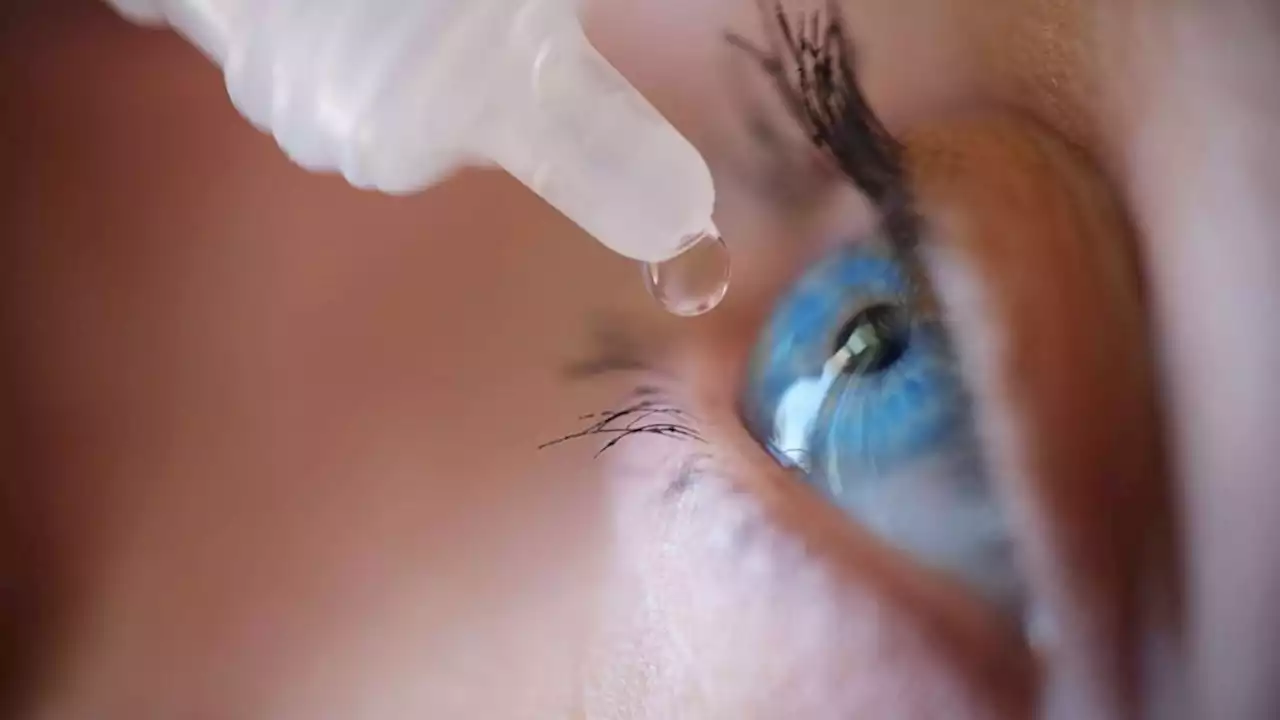 Be wary of unproven eye drop treatment with amniotic fluid, FDA saysThe FDA is warning Americans to stay away from eye drops treatments that contain amniotic fluid, saying there is no evidence they are safe or effective.
Be wary of unproven eye drop treatment with amniotic fluid, FDA saysThe FDA is warning Americans to stay away from eye drops treatments that contain amniotic fluid, saying there is no evidence they are safe or effective.
Read more »
 Alzheimer's: Medicare will cover Leqembi for all patients if FDA approves drug, CMS chief saysMedicare has severely restricted access to the medication Leqembi, which costs $26,500 per year, because the FDA approved it just on an expedited basis.
Alzheimer's: Medicare will cover Leqembi for all patients if FDA approves drug, CMS chief saysMedicare has severely restricted access to the medication Leqembi, which costs $26,500 per year, because the FDA approved it just on an expedited basis.
Read more »
