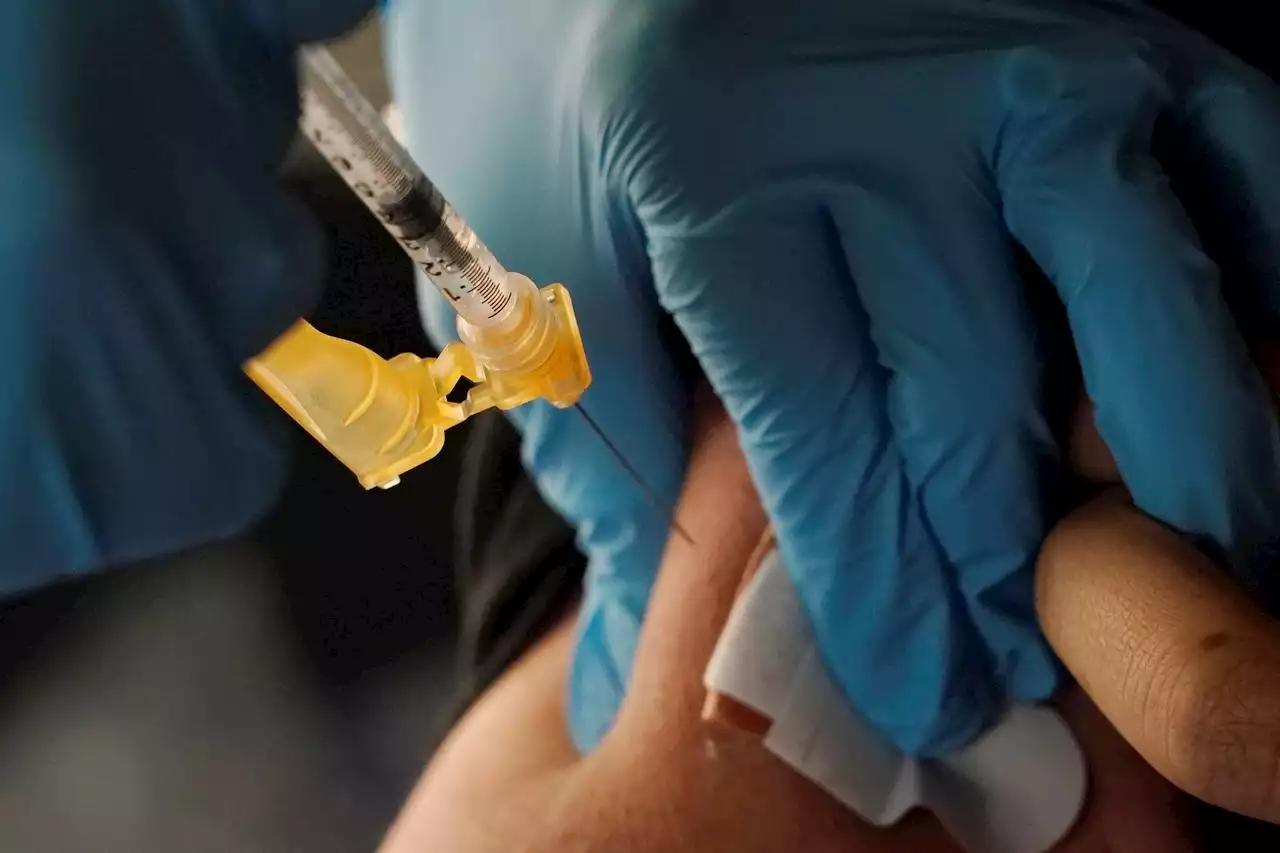
The CDC signed off on a more flexible booster schedule for people who remain at the highest risk from COVID-19.
A nurse administers a Moderna COVID-19 booster vaccine at an inoculation station next to Jackson State University in Jackson, Miss., Friday, Nov. 18, 2022. U.S. regulators on Tuesday, April 18, 2023, cleared another COVID-19 booster dose for older adults and people with weak immune systems so they can shore up protection this spring — while taking steps to make coronavirus vaccinations simpler for everyone else.
The move came a day after the Food and Drug Administration took steps to make coronavirus vaccinations simpler for everyone. From now on, anyone getting a Pfizer or Moderna dose — whether it’s a booster or their first-ever vaccination — will get an updated version rather than the outdated original shots.Anyone who’s gotten their original vaccinations but hasn’t had an updated booster yet can still get one.
The changes put the U.S. in line with Britain and Canada, which also are offering certain vulnerable populations a spring shot. It’s a reasonable choice, Dr. Matthew Laurens, of the University of Maryland School of Medicine, said before the announcement.
Malaysia Latest News, Malaysia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Extra spring COVID booster cleared for certain AmericansCertain Americans at high risk from COVID-19 can get an extra vaccine booster this spring
Extra spring COVID booster cleared for certain AmericansCertain Americans at high risk from COVID-19 can get an extra vaccine booster this spring
Read more »
 Extra spring COVID booster cleared for certain AmericansThe FDA said cleared a second booster for anyone 65 or older as long as it’s been at least four months since their first dose.
Extra spring COVID booster cleared for certain AmericansThe FDA said cleared a second booster for anyone 65 or older as long as it’s been at least four months since their first dose.
Read more »
 Extra spring COVID booster cleared for certain AmericansCertain Americans at high risk of becoming seriously ill from COVID-19 will be able to get an extra bivalent vaccine booster this spring, the FDA says.
Extra spring COVID booster cleared for certain AmericansCertain Americans at high risk of becoming seriously ill from COVID-19 will be able to get an extra bivalent vaccine booster this spring, the FDA says.
Read more »
 Extra spring COVID booster cleared for certain AmericansCertain Americans at high risk from COVID-19 can get an extra vaccine booster this spring. The Food and Drug Administration is allowing an extra dose of the omicron-targeted vaccine for anyone 65 and older if it's been four months since their last shot. Most people with weak immune systems can choose another shot at least two months later. The FDA also said Tuesday that the original versions of the Pfizer and Moderna shots are outdated and only the newer combo shots that add omicron protection will be used for everyone.
Extra spring COVID booster cleared for certain AmericansCertain Americans at high risk from COVID-19 can get an extra vaccine booster this spring. The Food and Drug Administration is allowing an extra dose of the omicron-targeted vaccine for anyone 65 and older if it's been four months since their last shot. Most people with weak immune systems can choose another shot at least two months later. The FDA also said Tuesday that the original versions of the Pfizer and Moderna shots are outdated and only the newer combo shots that add omicron protection will be used for everyone.
Read more »
 Extra spring COVID-19 booster shot cleared for certain Americans'At this stage of the pandemic, data supports simplifying the use' of the Pfizer and Moderna vaccines, FDA vaccine chief Dr. Peter Marks said
Extra spring COVID-19 booster shot cleared for certain Americans'At this stage of the pandemic, data supports simplifying the use' of the Pfizer and Moderna vaccines, FDA vaccine chief Dr. Peter Marks said
Read more »
 Extra spring COVID-19 booster shot cleared for certain Americans'At this stage of the pandemic, data supports simplifying the use' of the Pfizer and Moderna vaccines, FDA vaccine chief Dr. Peter Marks said
Extra spring COVID-19 booster shot cleared for certain Americans'At this stage of the pandemic, data supports simplifying the use' of the Pfizer and Moderna vaccines, FDA vaccine chief Dr. Peter Marks said
Read more »