The FDA issued a safety communication earlier this month about amniotic fluid eye drops being improperly marketed for dry eye disease.
It's not clear if the companies made other changes to their products or their marketing following the FDA's earlier letters. The
"Your product is not the subject of an approved biologics license application , nor is there an IND in effect for your product," the FDA stated in both letters.getting tougher "The problem with such commercial activity is that such companies haven't tested their products in controlled clinical trials and the safety and efficacy of such amniotic fluid products in the treatment of individuals with dry eye disease and other diseases have not yet been established," Turner told MedPage Today. "These untested or inadequately tested products pose risks to patients, as the recent public safety notification issued by the FDA notes.
Paul Knoepfler, of the University of California Davis, who has also been tracking the stem cell industry, said that these products still could contain growth factors and other substances that could have activity in the eyes.
Malaysia Latest News, Malaysia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA grants accelerated approval to Biogen treatment for rare form of ALSThe FDA has deemed Biogen's torferson reasonably likely to produce a clinical benefit for patients.
FDA grants accelerated approval to Biogen treatment for rare form of ALSThe FDA has deemed Biogen's torferson reasonably likely to produce a clinical benefit for patients.
Read more »
 Outdoor dining with your dog is safe, according to the FDAAccording to this new FDA guidance, you can have your bark with your bite.
Outdoor dining with your dog is safe, according to the FDAAccording to this new FDA guidance, you can have your bark with your bite.
Read more »
 Kamala Harris says wrong name for FDA in interview about mifepristoneHarris mistakenly said the Federal Drug Administration approved the abortion pill mifepristone more than two decades ago — but such an agency doesn’t exist.
Kamala Harris says wrong name for FDA in interview about mifepristoneHarris mistakenly said the Federal Drug Administration approved the abortion pill mifepristone more than two decades ago — but such an agency doesn’t exist.
Read more »
 Foghorn Therapeutics stock slides 21% after FDA puts partial clinical hold on cancer drug trialFoghorn Therapeutics Inc.’s stock slid 21% in premarket trade Monday, after the biotech said the Food and Drug Administration has placed a partial hold on a...
Foghorn Therapeutics stock slides 21% after FDA puts partial clinical hold on cancer drug trialFoghorn Therapeutics Inc.’s stock slid 21% in premarket trade Monday, after the biotech said the Food and Drug Administration has placed a partial hold on a...
Read more »
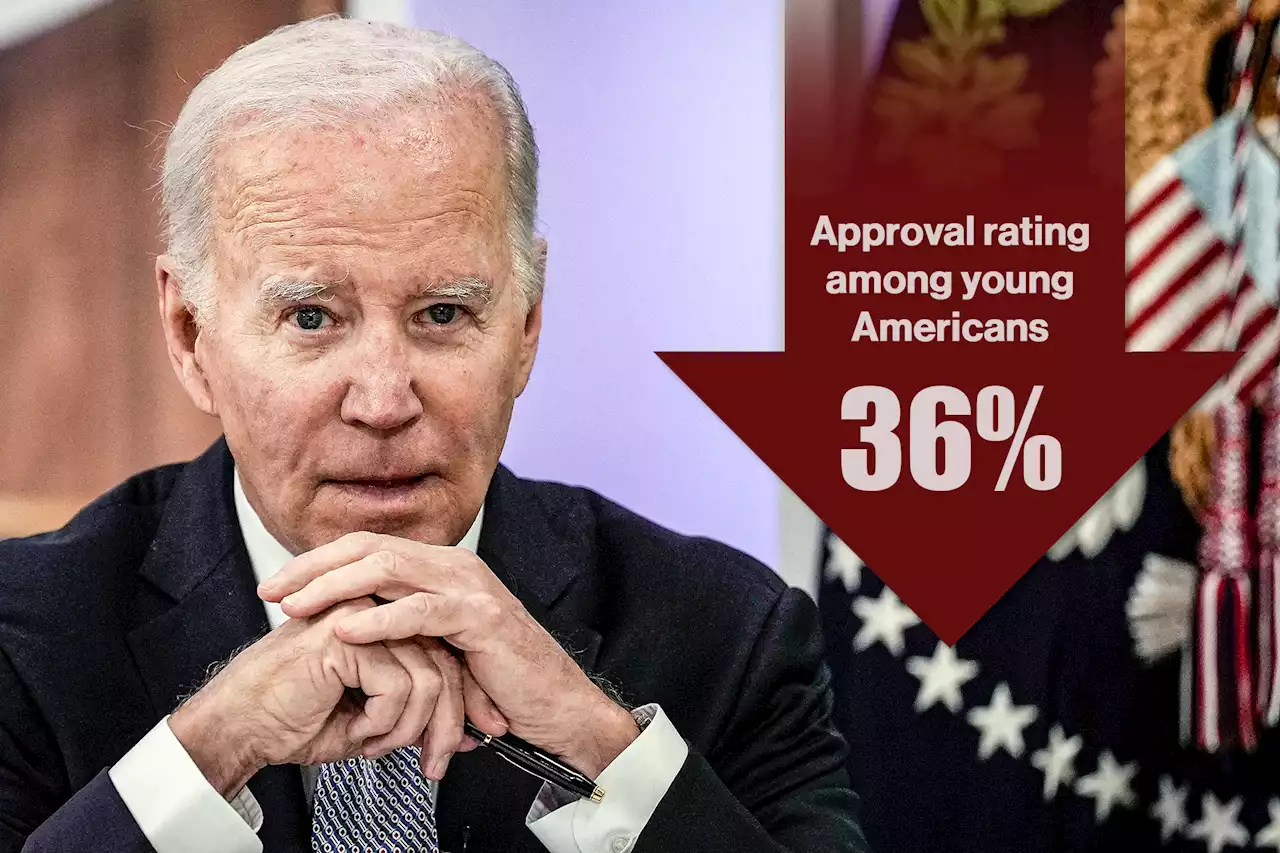 Biden’s approval rating drops among young Americans to 36%: pollThe national poll, put out by the Institute of Politics at Harvard Kennedy School, found 36% of registered voters aged between 18 and 29 years currently approve of the 80-year-old president’s…
Biden’s approval rating drops among young Americans to 36%: pollThe national poll, put out by the Institute of Politics at Harvard Kennedy School, found 36% of registered voters aged between 18 and 29 years currently approve of the 80-year-old president’s…
Read more »
 FDA Okays Latest Artificial Pancreas, the MiniMed 780GThe updated automated insulin delivery system features so-called meal detection technology and 7-day wear for the insulin pump.
FDA Okays Latest Artificial Pancreas, the MiniMed 780GThe updated automated insulin delivery system features so-called meal detection technology and 7-day wear for the insulin pump.
Read more »
